
What is the coverage for Xeljanz?
XELJANZ is covered for 99% of commercially-insured, moderate to severe rheumatoid arthritis patients with a prescription. †‡ * The Pfizer Patient Assistance Program is a joint program of Pfizer Inc. and the Pfizer Patient Assistance Foundation™.
How do I contact Xeljanz?
Phone 1-844-XELJANZ (1-844-935-5269) • Fax 1-866-297-3471 • 2730 S. Edmonds Lane, Suite 300, Lewisville, TX 75067 Patient Declaration– By signing below, I affirm that my answers and my proof-of-income documents are complete, true, and accurate to the best of my knowledge.
Is Xeljanz XR safe to take?
XELJANZ/XELJANZ XR should be used with caution in patients who may be at increased risk for gastrointestinal perforation (e.g., patients with a history of diverticulitis). LABORATORY ABNORMALITIES Lymphocyte Abnormalities
How to use xelsource patient portal?
Get the Xelsource Patient Portal you need. Open it using the online editor and begin altering. Fill in the blank areas; engaged parties names, addresses and numbers etc. Customize the blanks with exclusive fillable fields.

How does Xeljanz work?
How XELJANZ Works. XELJANZ is a Janus kinase (JAK) inhibitor that helps disrupt JAK pathways from inside the cells, which are believed to play a role in inflammation. Watch to learn more about the science of XELJANZ and how JAK inhibitors are thought to work. Video Player is loading. Play Video.
What is Xeljanz used for?
XELJANZ (tofacitinib) is the first and only pill of its kind (JAK inhibitor) that treats adults with moderate to severe rheumatoid arthritis, active psoriatic arthritis, and moderate to severe ulcerative colitis. Getting Started With XELJANZ. I'm Ready To Talk.
When was Xeljanz approved?
XELJANZ has been prescribed for more than 8 years, since initial approval in 2012, for adults with moderate to severe rheumatoid arthritis. XELJANZ was approved to treat adults with active psoriatic arthritis in 2017 and adults with moderate to severe ulcerative colitis in 2018.
What is the phone number for Xeljanz?
To speak to someone at XELSOURCE about your insurance coverage and medication costs for XELJANZ or XELJANZ XR, Monday through Friday, 8:00 AM - 8:00 PM ET, call 1-844-935-5269 and say “Representative.”.
How to speak with someone at XELSOURCE?
To speak with someone at XELSOURCE about your insurance coverage and medication costs for XELJANZ or XELJANZ XR , call 1-844-935-5269 and say “Representative.”. XELJANZ is covered for 98% of commercially-insured, moderate to severe rheumatoid arthritis patients with a prescription. †‡.
Can XELSOURCE change your health insurance?
Health plans can change—so can your health insurance and prescription benefits. Once your new plan begins, XELSOURCE can help review your plan information, verify your coverage if needed, and assist with the Prior Authorization (PA) process.
1 INDICATIONS AND USAGE
XELJANZ/XELJANZ XR is indicated for the treatment of adult patients with moderately to...
3 DOSAGE FORMS AND STRENGTHS
5 mg tofacitinib: White, round, immediate-release film-coated tablets, debossed with "Pfizer" on one...
5 WARNINGS AND PRECAUTIONS
Serious and sometimes fatal infections due to bacterial, mycobacterial, invasive fungal, viral, or other...
6 ADVERSE REACTIONS
The following clinically significant adverse reactions are described elsewhere in the labeling:
7 DRUG INTERACTIONS
Table 6 includes drugs with clinically important drug interactions when administered concomitantly with XELJANZ/XELJANZ XR/XELJANZ Oral Solution and instructions for preventing or managing them.
8 USE IN SPECIFIC POPULATIONS
All information provided in this section is applicable to XELJANZ/XELJANZ XR/XELJANZ Oral Solution as they contain the same active ingredient (tofacitinib).
10 OVERDOSAGE
There is no specific antidote for overdose with XELJANZ/XELJANZ XR/XELJANZ Oral Solution. In case of an overdose, it is recommended that the patient be monitored for signs and symptoms of adverse reactions....
How to fill out and sign xelsource patient portal com online?
Get your online template and fill it in using progressive features. Enjoy smart fillable fields and interactivity. Follow the simple instructions below:
Accredited Business
Guarantees that a business meets BBB accreditation standards in the US and Canada.
What are the most common adverse reactions to Xeljanz?
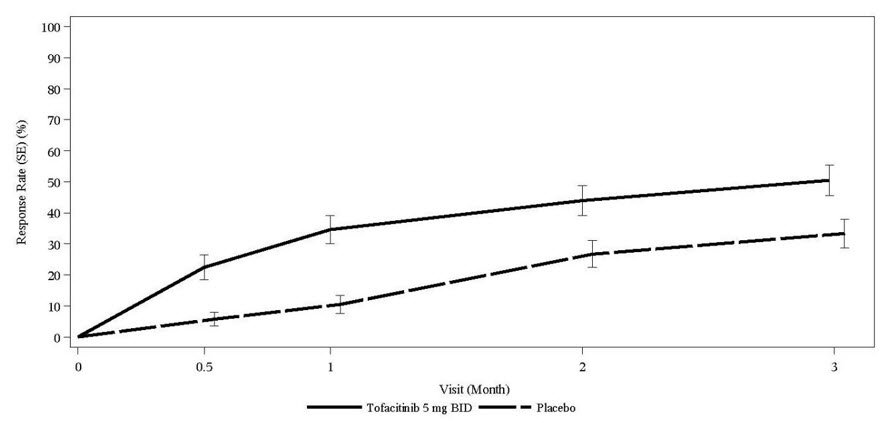
The most commonly reported adverse reactions during the first 3 months in controlled clinical trials with XELJANZ 5 mg twice daily and placebo, respectively, (occurring in greater than or equal to 2% of patients treated with XELJANZ with or without DMARDs) were upper respiratory tract infections (4.5%, 3.3%), headache (4.3%, 2.1%), diarrhea (4.0%, 2.3%), and nasopharyngitis (3.8%, 2.8%).
What does XELJANZ XR look like?
When you take XELJANZ XR, you may see something in your stool that looks like a tablet. This is the empty shell from the tablet after the medicine has been absorbed by your body.
Can XELJANZ XR be administered with strictures?
Caution should be used when administering XELJANZ XR to patients with pre-existing severe gastrointestinal narrowing. There have been rare reports of obstructive symptoms in patients with known strictures in association with the in gestion of other drugs utilizing a non-deformable extended release formulation.
Does XELJANZ inhibit gastrointestinal perforation?
Gastrointestinal perforations have been reported in XELJANZ clinical trials, although the role of JAK inhibition is not known. XELJANZ/XELJANZ XR should be used with caution in patients who may be at increased risk for gastrointestinal perforation (e.g., patients with a history of diverticulitis).
Can Xeljanz cause TB?
XELJANZ/XELJANZ XR may cause serious side effects, including:Serious infections. XELJANZ/XELJANZ XR can lower the ability of your immune system to fight infections. Some people can have serious infections while taking XELJANZ/XELJANZ XR, including tuberculosis (TB), and infections caused by bacteria, fungi, or viruses that can spread throughout the body. Some people have died from these infections. Your healthcare provider should test you for TB before starting and during XELJANZ/XELJANZ XR treatment, and monitor you closely for signs and symptoms of TB infection during treatment. You should not start taking XELJANZ/XELJANZ XR if you have any kind of infection unless your healthcare provider tells you it is okay.
Is tofacitinib safe for pregnant women?
There are no adequate and well-controlled studies in pregnant women and the estimated background risks of major birth defects and miscarriage for the indicated population is unknown. Based on animal studies, tofacitinib has the potential to affect a developing fetus. Women of reproductive potential should be advised to use effective contraception.
Popular Posts:
- 1. great falls clinic patient portal login
- 2. pro health patient portal
- 3. palmetto medical group patient portal
- 4. john randolph patient portal
- 5. health services patient portal
- 6. trivalley patient portal
- 7. scoi patient portal
- 8. patient portal adena
- 9. professional physical therapy patient portal
- 10. blue sky patient portal